Automating Compliance with Generative AI
GlobalComplyAI builds AI-powered platforms that automate regulatory compliance across regulated industries, transforming compliance into a continuous, intelligent system.

GlobalComplyAI builds AI-powered platforms that automate regulatory compliance across regulated industries, transforming compliance into a continuous, intelligent system.

GlobalComplyAI uses Generative AI to automate compliance processes across regulated industries. We started with medical laboratories and are expanding into other domains where regulatory compliance is critical.
Our vision is a world where compliance is not a burden but an intelligent, continuous system that protects organizations and the people they serve.
Discover How It Works
We envision compliance into an automated, intelligent, and always-on.
Purpose-built solutions for regulated industries
AI-powered compliance automation for medical laboratories (CLIA, CAP, ISO 15189). Automate document mapping, detect evidence gaps, and maintain continuous inspection readiness.
Compliance automation for pharmaceutical manufacturing. FDA 21 CFR Part 11, GMP, and quality management system support.
Regulatory compliance automation for financial services. SOX, AML, KYC, and banking regulation support.
A leadership team combining deep compliance expertise, enterprise execution, and AI-driven innovation.
Founder & CEO
Jay Wang is the Founder and CEO of GlobalComplyAI, leading the vision and architecture of ComplyGen Lab™, an AI-powered compliance automation platform for regulated industries.
Chief Operating Officer
Shiping Wang serves as Chief Operating Officer at GlobalComplyAI, overseeing execution, operations, and product delivery.
Chief Commercial Officer
Greg Cox is Chief Commercial Officer at GlobalComplyAI, responsible for go-to-market strategy, partnerships, and commercial growth.
Strategic Advisor & Board Member
Jason Klimpel is a Strategic Advisor and Board Member at GlobalComplyAI, contributing industry insight, customer access, and market strategy.
Board Member & Advisor
Dale Tweedy is a Board Member and Advisor to GlobalComplyAI, providing strategic guidance on scaling, governance, and long-term value creation.
![[background image] image of a futuristic laboratory setting (for a ai biotech company)](images/f2e07a9b-8eab-4e43-9503-0f40262895a4.avif)
Eliminate hours of manual document review. AI handles the heavy lifting while your team focuses on high-value tasks.
Generate comprehensive compliance reports instantly. No more last-minute scrambles before inspections.
AI links SOPs, policies, and forms to CLIA, CAP, and ISO 15189 standards. No more manual tracking or missed connections.
Instantly spot compliance gaps. Get notified about missing documents, outdated SOPs, or incomplete records before inspections.
Track compliance status across all locations. Access readiness scores, exportable reports, and audit trails anytime.
Get clear recommendations to resolve issues. Track progress and keep a full audit history.
Medical Laboratory Compliance dashboard designed for lab leadership
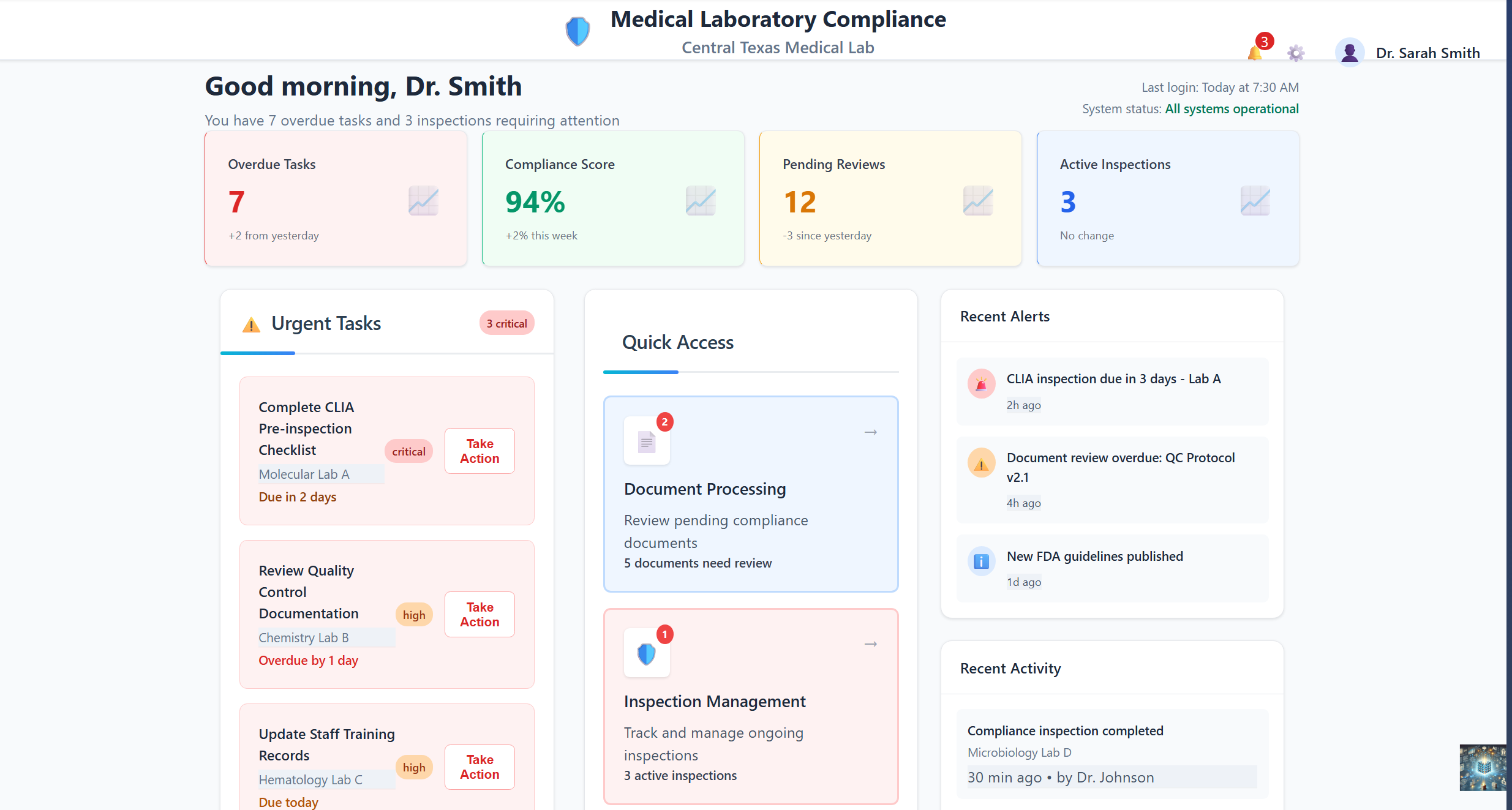
A real-time overview of compliance health, highlighting overdue and urgent tasks, overall compliance score, pending reviews, and active inspections. The interface emphasizes proactive risk management with alerts for upcoming CLIA inspections, document reviews, and regulatory updates.
For Lab Directors: Prioritize actions and maintain continuous compliance efficiently with proactive risk management alerts for regulatory updates.

Traditional compliance approaches drain resources—staff spend countless hours organizing documents, cross-referencing requirements, and preparing for inspections.
Our AI eliminates these bottlenecks by learning your organization's document structure and automatically maintaining alignment with evolving regulations.
The result: your team reclaims valuable time while maintaining higher compliance standards than ever before.

Explore how AI is reshaping compliance management across healthcare and beyond.
![[digital project] image of a case file on a tablet with legal tech elements](images/7a5f22aa-d865-4280-a069-dbada5c8463e.avif)
Give stakeholders confidence with verifiable compliance documentation.

Maintain consistent standards across multiple sites with centralized oversight.
Securely track all compliance actions in one place.
Enhance SOPs and policies for seamless inspections.
Resolve compliance gaps with step-by-step guidance.
Stay informed when standards are updated with automatic notifications.
Follow essential steps to prepare for inspections.
Get clarity on compliance automation and best practices.
Upload documents in any format
AI analyzes and categorizes content
Receive prioritized action items
Trusted by top medical labs
Feedback from compliance professionals

GlobalComplyAI made audit prep seamless and inspections stress-free.

Automation cut our compliance workload and improved accuracy.
![[background image] image of busy office environment (for an hr tech)](images/710d2b01-cb02-4629-8706-352b3a420ad7.avif)
Real-time dashboards keep us inspection-ready every day.
Deficiencies dropped and document quality improved across sites.

ComplyGen Lab centralized our audit trail and simplified evidence tracking.
![[headshot] image of satisfied customer shaking hands with company representative (for a fintech company)](images/fd68d6f8-070e-49a9-a8ef-2ae810c01b0e.avif)
Automated mapping to CLIA and CAP saved our team hours.

Corrective action guidance made compliance much easier.

We’re always ready for inspections with less manual effort.
Answers to common questions about automated compliance, supported standards, data security, and onboarding for medical labs.
AI analyzes your lab documents, matches them to regulatory requirements, and monitors for compliance gaps. You get real-time insights and reduced manual workload.
Supports CLIA, CAP, and ISO 15189. Additional frameworks can be added to meet your lab's needs.
Your data stays private and secure with HIPAA-aligned practices, encryption, and strict access controls. Data is never used to train public AI models.
Provide your lab's documents—SOPs, QC data, policies. The platform organizes and maps them to rules, with onboarding support from our team.

At GlobalComplyAI, we believe your data belongs to you—always. All documents and data you upload are managed and owned exclusively by you.
All data and documents uploaded to our platform remain your exclusive property. You maintain full ownership at all times.
No one else can access your data—not us, not third parties. Your documents are protected by enterprise-grade encryption.
Our platform is built with privacy at its core. You control who sees your data and can delete it anytime.
"Your compliance data contains sensitive business information. That's why we've built our platform so that all client data and documents are managed and owned exclusively by you—no exceptions. No one at GlobalComplyAI or any third party can access your information without your explicit permission."
Manage your lab compliance checklists and inspection document templates
Upload and manage checklists tailored to your lab environment. Your lab maintains full control over checklist content and updates.
Lab Ownership: Your lab is responsible for managing checklist content, ensuring it stays current with your specific compliance requirements.
Access pre-built inspection templates or create your own. Streamline documentation for audits and regulatory reviews.
Flexible Options: Choose from our library of regulatory-compliant templates or upload documents tailored to your lab's specific inspection requirements.
Find resources, documentation, and support to get the most out of GlobalComplyAI
New to GlobalComplyAI? Learn the basics and set up your first compliance project.
Detailed guides and references for all platform features and integrations.
Need help? Our support team is here to assist you with any questions.
Our team is ready to help you with any compliance questions.
Contact UsQuestions? Our team is ready to help atcontact@globalcomplyai.com
